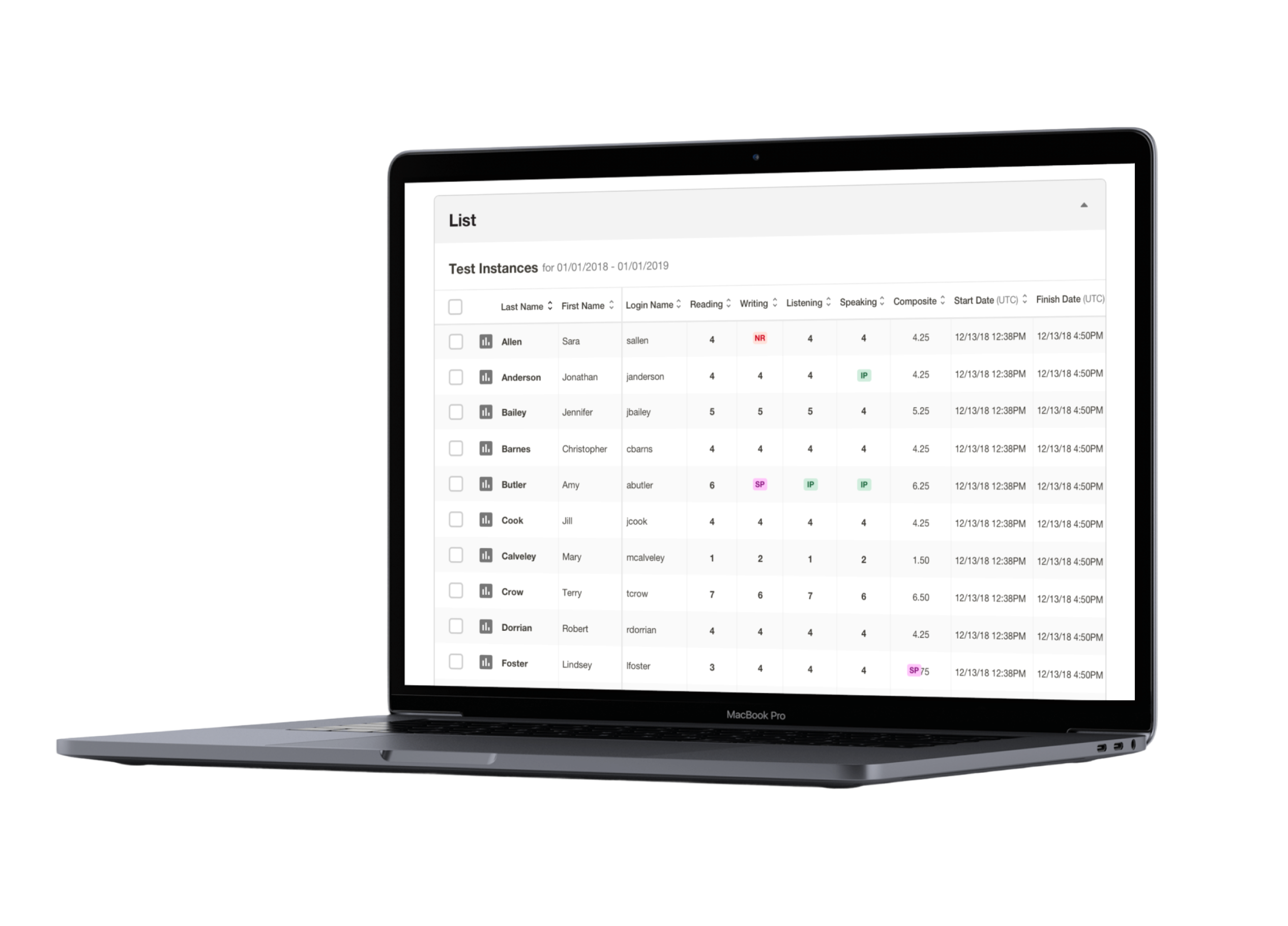
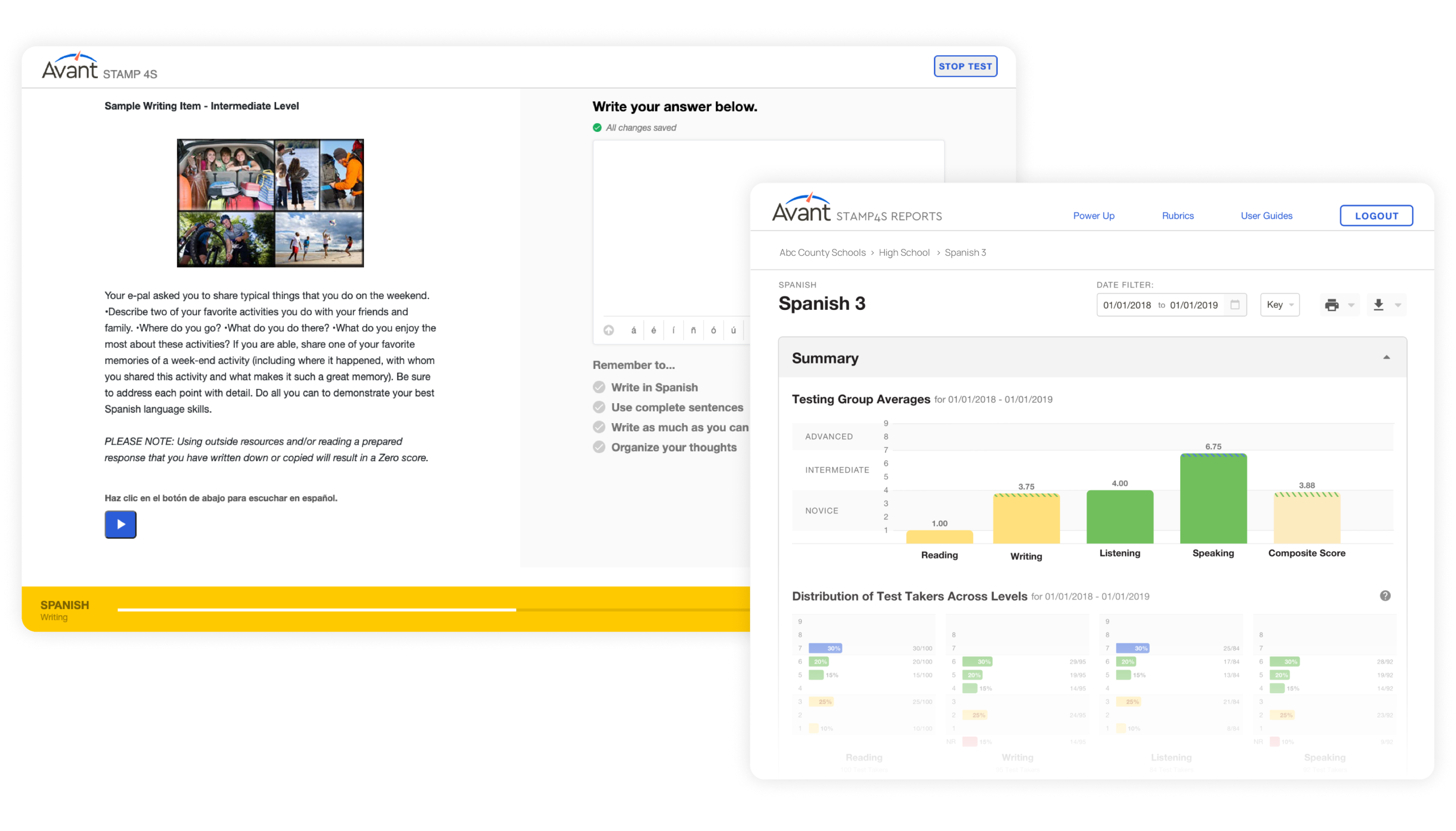
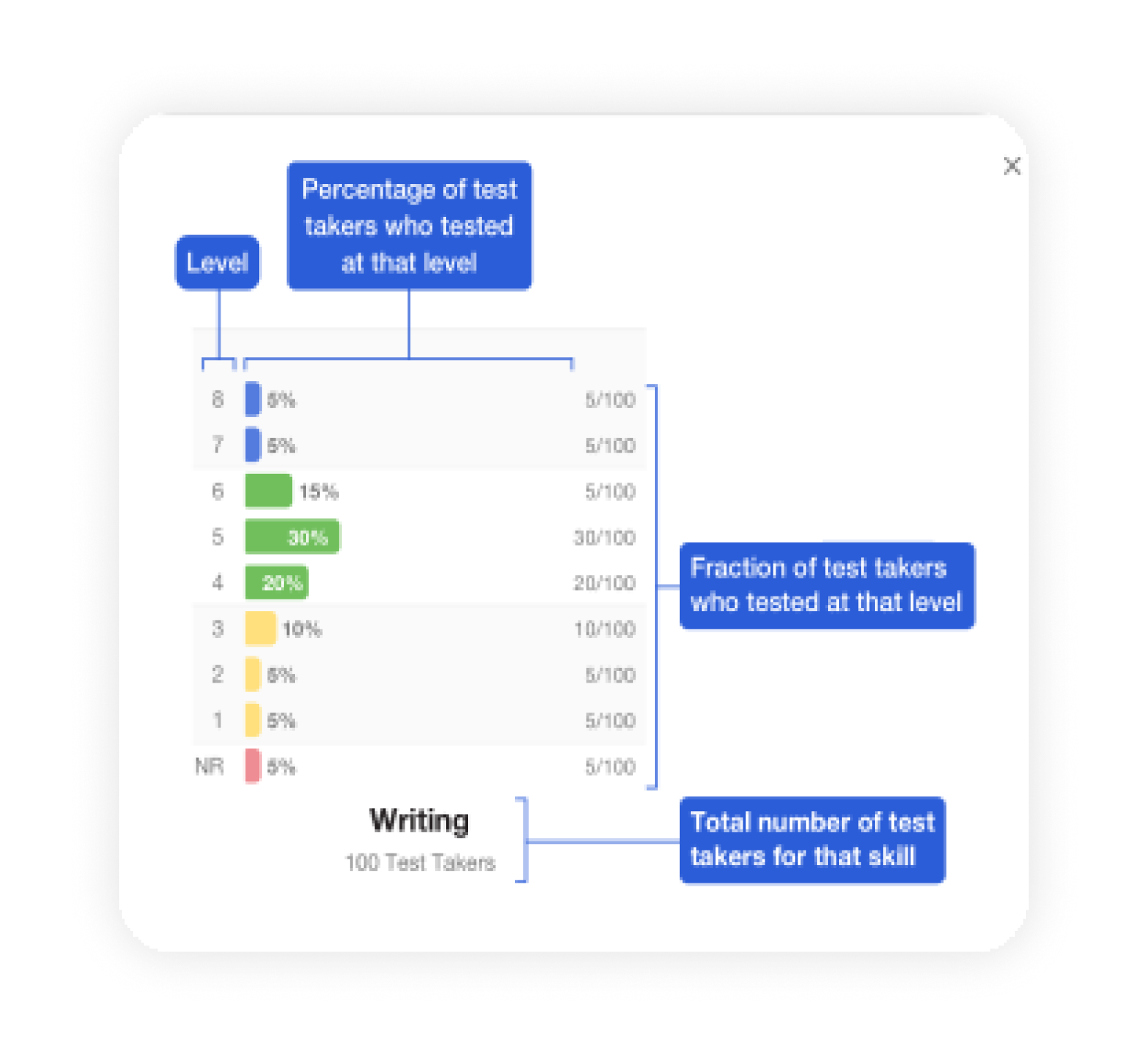
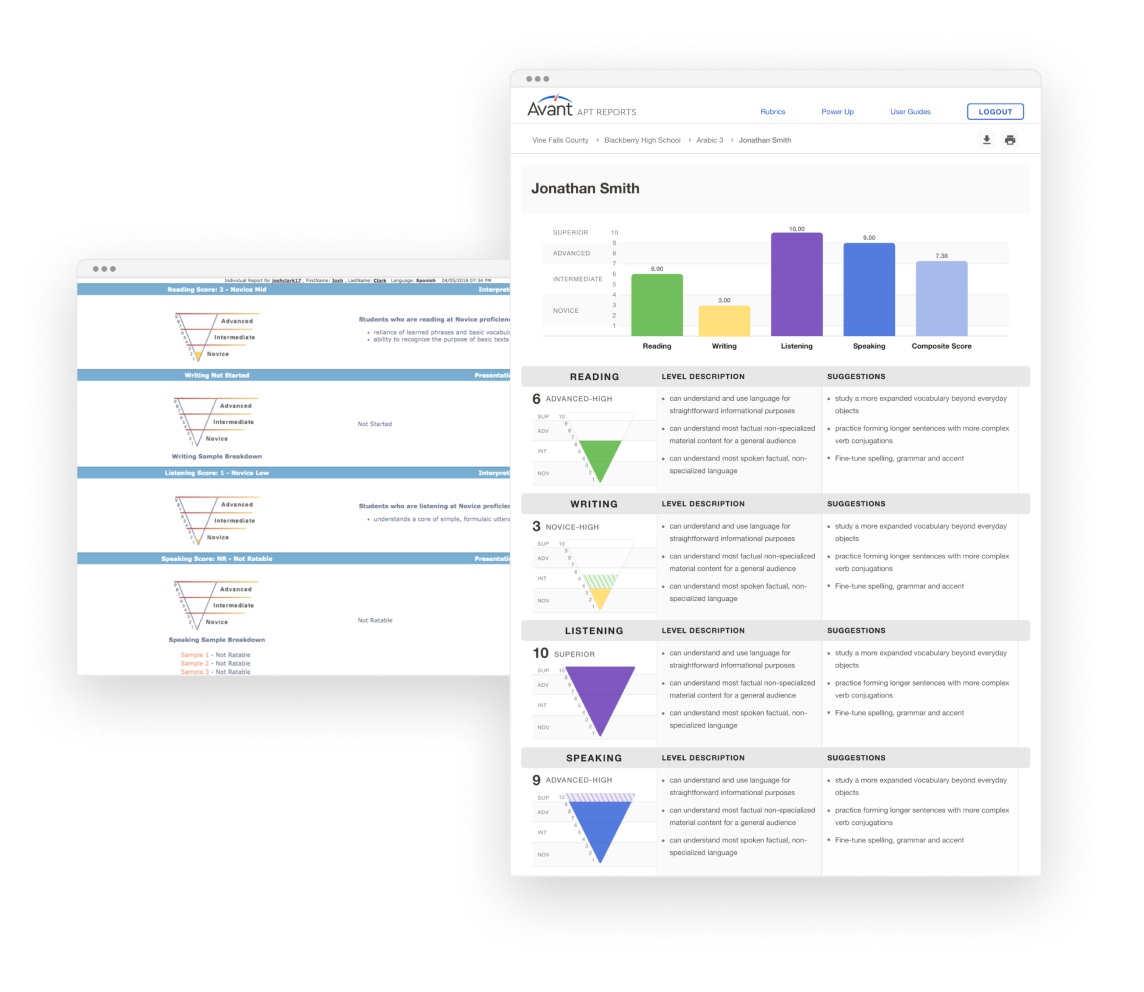
Avant Assessment, developers of the first internationally recognized online language testing standard (STAMP), tapped into a massive demand for their services and quickly moved to expand the variety and capacity of their products. They earned the trust of educators, employers, and government agencies across the world, but unfortunately, their rapid growth outpaced their technological infrastructure.